Stockholm/Lugano, April 25th, 2017 • It is a pleasure to share the latest diffusion study results on the AKVANO® drug delivery system developed and owned by our partner Lipidor AB (from now on LIPIDOR).
Results from the new in vitro permeation experiments, which have been performed on ketoprofen, diclofenac diethylamine and diclofenac sodium formulations in AKVANO®, can be seen in this white paper.
AKVANO® is a drug delivery technology based on sprayable formulations consisting of lipid components dissolved in a volatile, water-free solvent mixture. When this formulation is applied to the skin, the solvent evaporates and a non-occlusive lipid layer is formed in an immediate interaction with the skin surface. Earlier studies show that by choosing different lipids in the AKVANO vehicle, the skin barrier could either be strengthened or weakened, made evident by changes in transepidermal water loss (TEWL).
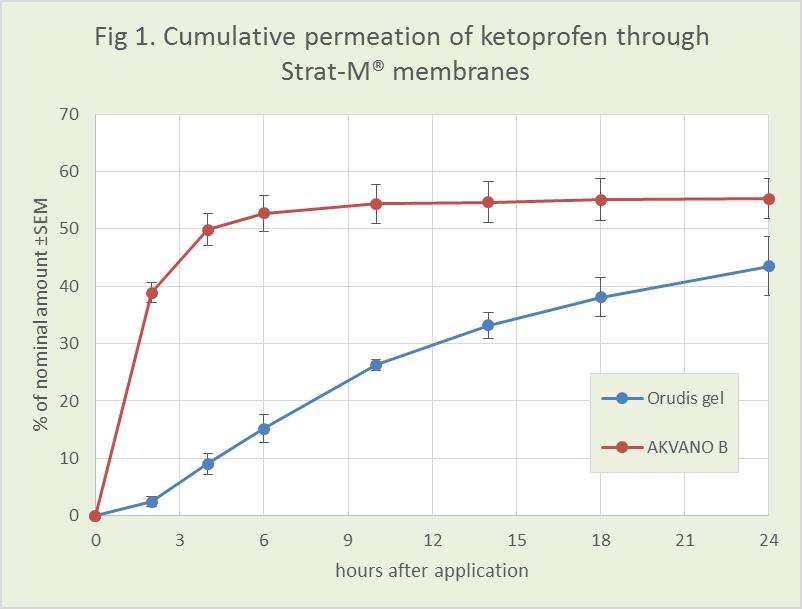
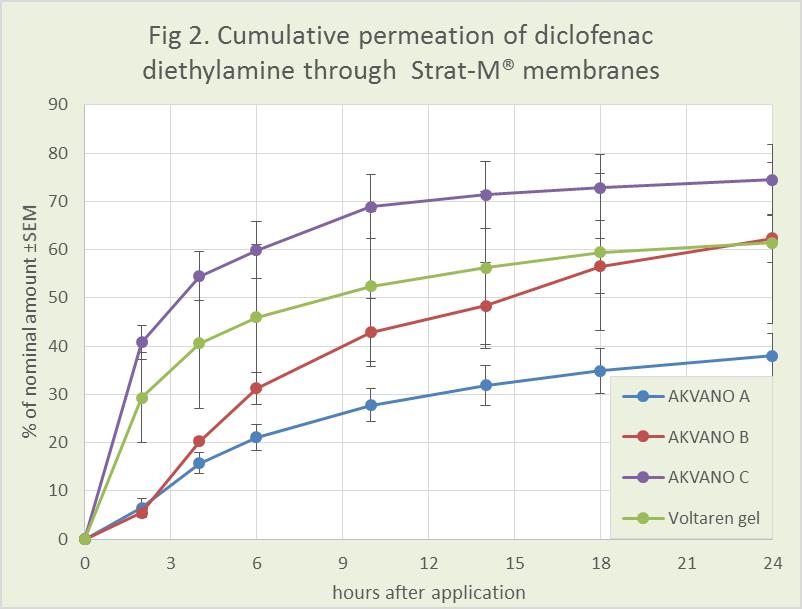
The new results show that by adjusting the composition of lipid components in AKVANO vehicles, it is possible to control permeation. By using a lipid composition with only barrier strengthening components, the penetration is relatively slow, and a significant part of the active substance is retained in or on the membrane. With a moderate amount of penetration enhancing lipids, the flux increases but registers a prolonged release profile. Finally, with a higher amount of the penetration enhancing lipids, the formulation gives a faster or equally fast release of the active substance through the membrane compared to commercial products Orudis® gel (2.5 % ketoprofen) and Voltaren® gel (2.3 % diclofenac diethylamine).
“I am very pleased with these results which confirm that AKVANO technology has the capacity to control the delivery rate of active components into the skin, and that it is possible to fine-tune the balance between penetration enhancement and strengthening of the protective skin barrier”, comments Lipidor’s CEO Anders Carlsson.
”We are very excited about the results obtained which confirm the very high flexibility of the AKVANO delivery system, not only in incorporating very different drug substances (from lipophilic to hydrophilic), but also providing a flexible delivery speed from fast to slow”, confirms Gabriel Haering, Cerbios’ CEO. “This gives very high flexibility for the development and manufacture of topical drugs currently at New Chemical Entities or Life-Cycle-Management stage”
About AKVANO
AKVANO® technology represents a completely new dosage form that creates a lipid layer on the skin surface for effective topical delivery of drugs and skin care treatment. AKVANO is available to companies wishing to innovate, develop or improve new and existing products in areas like pharmaceuticals, biocides, cosmetics and medical devices. So far, more than 80 active ingredients have been successfully incorporated into AKVANO, of which more than 50 are active pharmaceutical ingredients (APIs). AKVANO is designed for local treatment, not for systemic delivery. AKVANO® is a registered trademark of Lipidor AB.
About Lipidor AB
Lipidor AB is a Swedish lipid technology company offering unique lipid based formulation opportunities across dermatology, wound and skin care markets. The company was established in 2009. Research and development is conducted at Stockholm University and Karolinska Institutet Science Park in Stockholm. Lipidor is owned by its founders, Karolinska Development, AB, Cerbios-Pharma SA, Aurena Laboratories AB and private investors (http://www.lipidor.se/) .
About Cerbios-Pharma SA
Cerbios is a privately held company located in Lugano, Switzerland, that specializes in the development and manufacture of both chemical and biological APIs for its partners world-wide.
Exclusive, third-party manufacturing services are offered by the Chemical Division for HPAIs and by the Biological Division for monoclonal antibodies, recombinant proteins and pharma probiotics.
For more information please contact:
Cerbios-Pharma SA
Phone : +41 (0) 91 985 63 11
Fax : +41 (0) 91 985 63 25
Email : sales@cerbios.ch
Or use our online contact form.
Lipidor AB
Hornsbergs strand 49
SE-112 16 Stockholm
SWEDEN
email: anders.carlsson@lipidor.se

